Scope: The Cady group focuses on the unique interface between nanotechnology and biology. Research in our group falls into the following two general categories:
- Approaching nanotechnology from the biological world -
Nanoscale innovations and technologies from the biological world are harnessed to manipulate and control materials at the nanoscale or inspire unique electrical devices and circuits. Drawing knowledge from biological systems enables unique approaches to nanotechnological design, engineering, processing and manufacturing. - Approaching biology from the nanoscale -
Nanoscale phenomena, technologies or processes are used to study biology at its fundamental level – the nanoscale. Similarly, nanoscale devices, materials, or phenomena can be harnessed for therapeutics, diagnostics, medicine, pharmaceuticals, and many other biological applications.
Resistive Memory Devices (Memristors) for Neuromorphic, AI, and Rad-Hard Applications:
We have established an ongoing research program on resistive memory devices (aka: memristors). These metal-insulator-metal (MIM) devices behave similarly to neural synapses, as their “memory state” depends on the current and voltage history of the device. This is a good example of bioinspired/ biomimetic research, since the biological process of synapse formation is mimicked by a physical, electronic device. We have previously developed memristors as both non-volatile memory (NVM) elements, as well as devices to control the reconfigurability of CMOS circuits (for encryption applications). Our current work (supported by the Air Force Research Laboratory / AFRL) is focused on integrating memristors with CMOS circuits for neuromorphic computing applications, in collaboration with faculty at the University of Tennessee – Knoxville (Profs. Garrett Rose, James Plank, and Mark Dean). In this work, memristors serve as “synapses”, literally encoding the synaptic weight between neural connections in the circuits. To date we have developed a full 65nm CMOS/memristor hybrid chip design and are in the process of fabricating these devices in the SUNY Poly 300mm fabrication facility. This work will greatly expand our ability to demonstrate fully hybrid CMOS/memristor circuits, and will be a launching pad for follow-on projects.
In addition to developing memristors for neuromorphic applications, we are also working on memristors for radiation hardened (rad hard) applications, with a specific focus on tantalum oxide based devices. This work has been supported by NASA, through their graduate research fellowship program (which funds my graduate student, Joshua Holt). Our work in this area has enabled partnerships with Dr. Jean Yang-Scharlotta (Jet Propulsion Laboratory), and Dr. Matthew Marinella at Sandia National Labs. It also represents a novel area of research for my group, particularly in the realm of radiation testing/exposure for nanoelectronic devices. As part of this effort, we have developed resistive memory devices that are resistant to all but the most extreme radiation environments, which should be of interest for space exploration and other rad-hard applications.
Beyond the projects mentioned above, we have ongoing efforts to characterize the switching mechanism of our devices, to better enable modeling and simulation of memristors in complex circuits. We are also investigating methods (both fabrication methods and testing methods) that reduce the stochastic nature of memristor device performance. This will improve reliability of these devices and make the amenable to larger scale integration with complex CMOS circuits, processors, etc.
In 2018 we secured funding from the Semiconductor Research Corporation (SRC), NSF, and continued funding from the AFRL, all of which will support our ongoing program in resistive memory and neuromorphic applications.
 |
 |
|
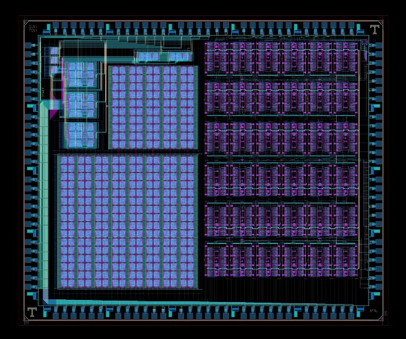
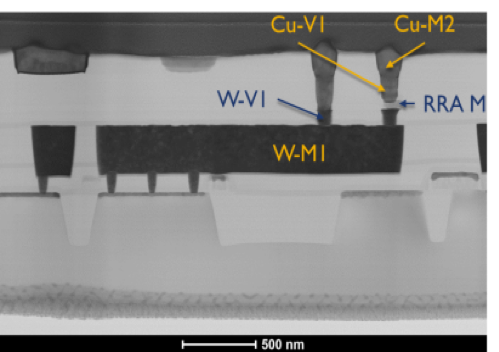
Figure 1: Layout of the “mrDANNA” CMOS-memristor neuromorphic chip designed in conjunction with UT-Knoxville and currently being fabricated by SUNY Poly (left). Cross-section of a hybrid CMOS-memristor 1T1R circuit fabricated in Prof. Cady’s group at SUNY Poly (right). |
|

Recent Publications:
- M. Uddin, M.B. Majumder, K. Beckmann*, H. Manem*, Z. Alamgir*, N.C. Cady, G.S. Rose. Design considerations for memristive crossbar physical unclonable functions. (2018) ACM Journal on Emerging Technologies in Computing Systems. 14(1), 2.
- N.C. Cady, K. Beckmann, W. Olin-Ammentorp*, J.E. Van Nostrand, G. Chakma, R. Weiss, S. Sayyaparaju, M. Adnan, J. Murray, M.E. Dean, J.S. Plank, G.S. Rose. Full CMOS-Memristor Implementation of a Dynamic Neuromorphic Architecture. GOMACTECH Conference, Miami, FL. March 2018.
- K. Beckmann, J. Holt, W. Olin-Ammentorp, Z. Alamgir, J. Van Nostrand, N.C. Cady. The effect of reactive ion etch (RIE) process conditions on ReRAM device performance. (2017) Semiconductor Science and Technology. 32: 095013
- Z. Alamgir, J. Holt, K. Beckmann, N.C. Cady. The effect of different oxygen exchange layers in TaOx based RRAM devices. (2017) Semiconductor Science & Technology. 33: 015014
- M. Uddin, M.B. Majumder, K. Beckmann, H. Manem, Z. Alamgir, N.C. Cady, G.S. Rose. Design considerations for memristive crossbar physical unclonable functions. (2017) ACM Journal on Emerging Technologies in Computing Systems. 14(1), 2.
- Z. Alamgir, K. Beckmann, J. Holt, N.C. Cady. Pulse width and height modulation for multi-level resistance in bi-layer TaOx based RRAM. Applied Physics Letters. (2017) 111: 063111 DOI: http://dx.doi.org/10.1063/1.4993058
- J.S. Plank, G.S. Rose, M. E. Dean, C.D. Schuman, N.C. Cady. A Unified Hardware/Software Co-Design Framework for Neuromorphic Computing Devices and Applications. ICRC: IEEE International Conference on Rebooting Computing. November 2017, Washington, DC.
- S. Amer, S. Sayyaparaju, K. Beckmann, N.C. Cady, G.S. Rose. A Practical Hafnium-Oxide Memristor Model Suitable for Circuit Design and Simulation. ISCAS: International Symposium on Circuits and Systems. May 2017, Baltimore, MD.
- S. Amer, G.S. Rose, K. Beckmann, N.C. Cady. Design Techniques for in-Field Memristor Forming Circuits. 60th IEEE International Midwest Symposium on Circuits and Systems. August 2017, Boston, MA.
- K. Beckmann, J. Holt, W. Olin-Ammentorp, J. Van Nostrand, N.C. Cady. Impact of etch process on hafnium dioxide based nanoscale RRAM devices. (2016) ECS Transactions. 75(13): 93-99.
- K. Beckmann, J. Holt, H. Manem, J. Van Nostrand, N.C. Cady. Nanoscale hafnium oxide RRAM devices exhibit pulse dependent behavior and multi-level resistance capability. (2016) MRS Advances. 1(49): 3355-3360. DOI: http://dx.doi.org/10.1557/adv.2016.377
- M. Uddin, M.B. Majumder, G.S. Rose, K. Beckmann, H. Manem, Z. Alamgir, N.C. Cady. Techniques for Improved Reliability in Memristive Crossbar PUF Circuits. 2016 IEEE Computer Society Annual Symposium on VLSI (ISVLSI), Pittsburgh, PA, pp. 212-217. doi: 10.1109/ISVLSI.2016.33
- G. Chakma, M.E. Dean, G.S. Rose, K. Beckman, H. Manem, N. Cady, A Hafnium-Oxide Memristive Dynamic Adaptive Neural Network Array. International Workshop on Post-Moore's Era Supercomputing (PMES), Salt Lake City, UT, November 2016.
- Z. Alamgir, K. Beckmann, N.C. Cady, A. Velasquez, S.K. Jha. Flow-based computing on nanoscale crossbars: design and implementation of full adders. International Symposium on Circuits and Systems (ISCAS) Conference, May 2016, Montreal, Canada.
- N.C. Cady, K. Beckmann, H. Manem, M.E. Dean, G.S. Rose, J.E. Van Nostrand. Towards Memristive Dynamic Adaptive Neural Network Arrays. GOMACTEC Conference, March 2016, Orlando, FL.
Biosensors & Microfluidics:
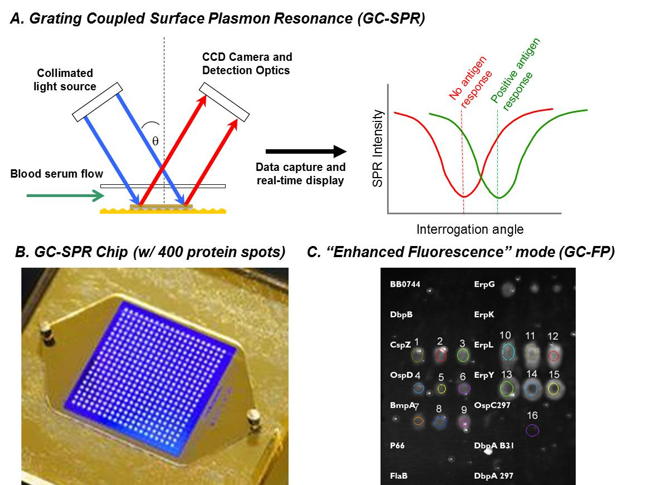
In 2018 our group has expanded our work on biosensors, primarily for the diagnosis of Lyme disease, which is caused by bites from ticks infected with the bacterium Borrelia burgdorferi. Diagnosis of this infection is currently expensive, time consuming, and non-quantitative. Working with our partners at Ciencia Inc. and the NYS Dept. of Health, we are developing a Lyme disease assay and platform that is based on grating coupled surface plasmon resonance (GC-SPR). We use GC-SPR in a unique format, namely grating-coupled fluorescent plasmonics (GC-FP mode). To date, we have demonstrated that this platform can detect a serological response to Lyme disease in mice. We have also been able to positively detect Lyme infection in human serum samples (as compared to gold-standard testing methods, such as ELISA and Western blot analysis). An example of how the system operates, and some sample results are shown in Figure 3, below.
In addition to working on assay development, we have also partnered with collaborators from the Unive. At Buffalo to model the plasmonic effects on our GC-SPR chips, and have published work on grating-coupled plasmonics for photocatalysis (with our partners at Ciencia Inc.).
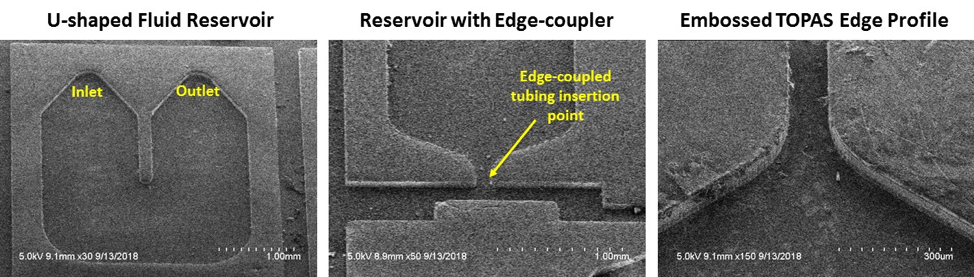
Beyond sensor-specific research, we are also working with the AIM Photonics program at SUNY Poly and collaborators at the Univ. of Rochester to develop microfluidic modules for silicon-photonic biosensors. Most of our efforts have been focused on high volume manufacturing compatible processes, including hot embossing. Working with our collaborators, we have shown that we can fabricate cyclic olefin-based polymers, such as TOPAS™ (Figure 4).
Recent Publications
- E. Chou, Y-P. Lin, N.C. Cady. Recent strategies for the diagnosis of early Lyme disease. (2018) Science Progress. 101(4): 311-331.
- J.F. Drazan, O.T. Abdoun, M.T. Wassick, R. Dahle, L.A. Beardslee, G.A. Marcus, N.C. Cady, E.H. Ledet. Simple Implantable Wireless Sensor Platform to Measure Pressure and Force. (2018) Medical Engineering & Physics. 59: 81-87.
- L. Shen, G.N. Gibson, N. Poudel, B. Hou, J. Chen, H. Shi, E. Guignon, N.C. Cady, W. Page, A. Pilar, S.B. Cronin. Plasmon Resonant Amplification of Hot Electron-Driven Photocatalysis. (2018) Applied Physics Letters. 113(11): 113104.
- B.P. Garreffi, M. Guo, N. Tokranova, N.C. Cady, J. Castracane, I.A. Levitsky. Highly Sensitive and Selective Fluorescence Sensor Based on Nanoporous Silicon-Quinoline Composite for Trace Detection of Hydrogen Peroxide Vapors. (2018) Sensors and Actuators B: Chemical. 276: 466-471.
- E. Chou, G. Zenteno, B. Taubner, A. Pilar, E. Guignon, W. Page, Y-P. Lin, N.C. Cady. Grating coupled-surface plasmon resonance and fluorescent plasmonics biosensor for diagnosis of Lyme disease. Proceedings of the SPIE. March 2018, Miami, FL.
- V. Sukhotskiy, N.C. Cady, E. Chou, I.V.A.K. Reddy, E.P. Furlani. Numerical modeling of a sinusoidal grating-based surface plasmon coupled emission biosensor. Nanotech 2018. Anaheim, CA.
- S. Nallanthighal, C. Chan, A.P. Mosier, N.C. Cady, R. Reliene. Differential effects of silver nanoparticles on DNA damage and DNA repair gene modulation in Ogg1 deficient and wildtype mice. (2017) Nanotoxicology. 11(8): 996-1011
- M.K. Dion, J.F. Drazan, S. Giddings, N.C. Cady, R. Dahle, J.T. Roberts, E.H. Ledet. Smart Orthopaedic Implants: Application in Total Knee Arthroplasty. (2016) American Journal of Engineering and Applied Sciences. 9(4):1232-38.
- A. Mendoza, D.M. Torrisi, S. Sell, N.C. Cady, D.A. Lawrence. Grating coupled SPR microarray analysis of proteins and cells in blood from mice with breast cancer. (2016) Analyst. 141: 704-712.
Antifouling and Biofilms:
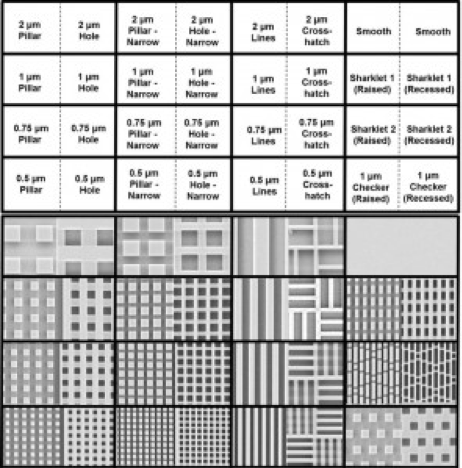
Microbial fouling of surfaces and subsequent formation of biofilms is a major problem in medicine, industrial processes, and infrastructure. To combat fouling and biofilm formation, we are pursuing methods to limit bacterial attachment to surfaces and interrupt biofilm formation (or disrupt established biofilms). For example, we have developed 3D nanomanufacturing strategies to create nanoscale topographical features that can be used to limit the attachment of bacterial cells to stationary surfaces. Our work has shown that topography in the 0.5 – 1 micrometer size scale is effective in reducing bacterial adhesion to surfaces, and that larger scale topography can increase surface attachment (as compared to flat reference surfaces).
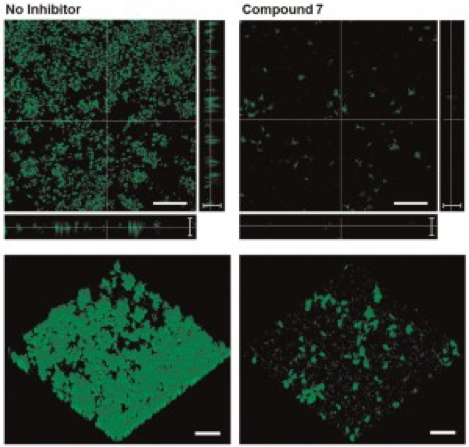
We are also working with collaborators (including Prof. Rabi Musah, UAlbany – Dept. of Chemistry) to develop molecular antagonists of biofilm formation and methods of delivering these antagonists for prophylactic or therapeutic treatment against biofilms. Our initial work in this area has focused on the inhibition of bacterial biofilm formation by a library of natural products inspired compounds. Prof. Musah’s group has developed these compounds, which we have shown to have efficacy against Pseudomonas aeruginosa biofilm formation. Interestingly, we also showed that these compounds are effective in reducing cell signaling (quorum sensing) behavior of P. aeruginosa. We recently demonstrated that one of our lead compounds (S-aryl cysteine sulfoxide) directly inhibits the enzyme kynureninase, which is linked to multiple pathways including those for quorum sensing autoinducer synthesis and various other virulence factors.
In addition to preventing biofouling and mitigating biofilm formation, we are also interested in developing methods to characterize biofilms and to utilize intact biofilms for various applications. To this end we have developed combined microfluidic / atomic force microscopy (AFM) based platforms to measure the mechanical properties of biofilms under varying fluidic conditions. We are also developing platforms to utilize biofilms for the treatment of metal-contaminated wastewater.
Related Recent Publications:
- S.H. Kasper, R. Hart, M. Bergkvist, R.A. Musah, N.C. Cady. Zein nanocapsules as a tool for surface passivation, drug delivery, and biofilm prevention. (2016) AIMS Microbiology. 2(4): 422-433.
- S.H. Kasper, R.P. Bonocora, J.T. Wade, R.A. Musah, N.C. Cady. Chemical inhibition of kynureninase reduces Pseudomonas aeruginosa quorum sensing and virulence factor expression. (2016) ACS Chemical Biology. 11(4): 1106-1117.
- M. Craven, S. Kasper, M. Canfield, R. Diaz-Morales, J. Hrabie, Joseph; N. Cady, A. Strickland. Nitric Oxide-Releasing Polyacrylonitrile Disperses Biofilms Formed by Wound-Relevant Pathogenic Bacteria. (2016) Journal of Applied Microbiology. 120(4): 1085-99.
- S. Kasper, D. Samarian, A. Jadhav, A. Rickard, R. Musah, N.C. Cady. S-Aryl-L-Cysteine Sulfoxides and Related Organosulfur Compounds Alter Oral Biofilm Development and AI-2 Based Cell-Cell Communication. (2014) Journal of Applied Microbiology. 117(5): 1472-86.
- A.P. Mosier, J. Behnke, E.T. Jin, N.C. Cady. Microbial biofilms for the removal of Cu2+ from CMP wastewater. Submitted to the Journal of Environmental Management, October 1, 2014.
- A.P. Mosier, S. Peters, M. Larsen, and N.C. Cady. Microfluidic platform for the characterization of mouse submandibular glands by atomic force microscopy. (2014) Biosensors. 4(1): 18-27.
- M.V. Graham, N.C. Cady. Nano and microscale topographies for the prevention of bacterial surface fouling. (2014) Coatings. 4(1): 37-59.
- M.V. Graham, A.P. Mosier, T.R. Kiehl, A.E. Kaloyeros, N.C. Cady. Development of antifouling surfaces to reduce bacterial attachment. (2013) Soft Matter. 9: 6235-6244.
- N.C. Cady, J. Behnke, R. Kubec, K. McKean, and R.A. Musah. Inhibition of Biofilm Formation, Quorum Sensing and Infection in Pseudomonas aeruginosa by Natural Products-Inspired Organosulfur Compounds. (2012) PLoS One. 7(6): e38492.
- J.F. Ling, M.V. Graham, N.C. Cady. Topographically patterned poly(dimethylsiloxane) surfaces affect Pseudomonas aeruginosa adhesion and biofilm formation. (2012) Nano LIFE. 2(4): 1242004.
- A.P. Mosier, A.E. Kaloyeros, N.C. Cady. A novel microfluidic device for the in situ optical and mechanical analysis of bacterial biofilms. (2012) Journal of Microbiological Methods. 91: 198-204.

